More and more, medical device manufacturers are replacing traditional components with smaller, lighter-weight options that have sufficient life and reliability for “life-critical” applications. One such example comes from manufacturers of ventilators, who are using voice coil actuators to control the valves that deliver air to a patient who’s unable to breathe on their own.
Ventilators are relatively simple devices, as explained by my colleague Lee Teschler in this post on our sister site, Test & Measurement Tips. They consist of around a dozen components: compressed air and oxygen tanks, connecting tubes, a set of valves (referred to as inspiratory and expiratory valves), a Y connection for connecting the tubes, sensors that monitor the air pressures and volumes through the breathing cycle, filters, a humidifier, and a ventilator mask for the patient.
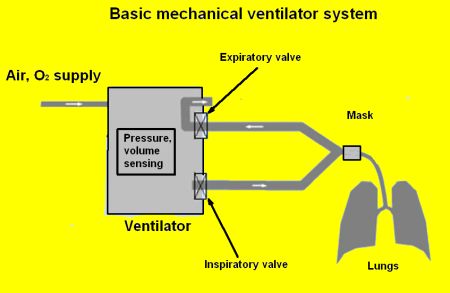
Despite their simplicity, however, ventilators are absolutely critical for patients who have compromised breathing due to a serious lung disease, surgery, or an illness, such as COVID-19, that causes severe respiratory problems.
Note that ventilators and respirators are different types of devices, although the terms are often used interchangeably. A respirator is type of face mask that protects a person from inhaling hazardous fumes, gases, microorganisms, or particles. A ventilator can employ a face mask or breathing tubes and moves air into and out of lungs for people unable to breathe on their own or who are breathing insufficiently.
As the cost of voice coil actuators has decreased in recent years, manufacturers have helped ensure their suitability for medical device applications through rigorous testing. In fact, one manufacturer — Sensata Technologies — has tested its BEI Kimco voice coil actuators for medical applications to ensure they can survive more than 100 million cycles (and counting).
But why would manufacturers — especially those dealing with critical medical equipment — switch from a tried-and-true technology, such as solenoids, to a (relatively) new solution, given strict testing and approval requirements? The driving factors are size and performance.

Image credit: Sensata Technologies
One benefit of voice coil actuators is that they’re relatively easy for manufacturers to customize to the exact application requirements, including the use of special materials for biocompatibility or other environmental requirements. They’re also available in either a moving coil design (better for acceleration) or a moving magnet design (better for heat dissipation). Regardless of the setup, they can be constructed with extremely small form factors. In fact, voice coil actuators as small as a thimble have been manufactured for some ventilator applications.
Voice coil technology also provides significantly better performance than solenoids — the traditional solution for valve control — in many respects. Their low moving mass gives them very high acceleration capabilities, and they provide precise, controllable, bi-directional motion, where solenoids are simply “on-off” devices and require a spring to provide bi-directional movements. And hysteresis in a voice coil actuator can be orders of magnitude lower than in a solenoid, meaning voice coils can provide much better repeatability. Of course, safety is also a significant concern in medical device applications. When voice coils are used to control air flow in ventilators, magnetic latches can be employed to ensure the actuator keeps valves open if power is lost, so the patient can continue to breathe on their own.





Leave a Reply
You must be logged in to post a comment.